PCC puts our proprietary technology and combustion processes know-how to work selecting the optimum burner(s), system configuration, process design, and operating conditions to meet the specified NOx emission limits. All NOx generation methods – “thermal NOx formation”, “chemical NOx formation” and “prompt NOx formation” are evaluated when determining whether low NOx burners, staged air systems, staged fuel systems and/or multi-stage combustion systems are required to achieve low NOx emissions.
In certain situations, combustion techniques, process changes and modifying operating conditions might not meet the specified NOx emission limits. In these case, NOx emissions can be met by adding one of two post-combustion NOx reduction processes:
- Selective Non-Catalytic Reduction (SNCR)
- Selective Catalytic Reduction (SCR)
When necessary, PCC incorporates the appropriate post-combustion NOx reduction technology (SNCR or SCR) into our thermal oxidizer systems. We custom design and supply the complete SNCR or SCR package. We can also design and supply “stand-alone” SNCR or SCR systems for new or existing combustion systems.
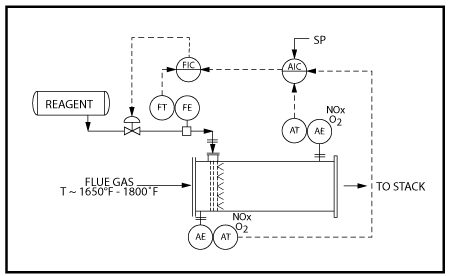
SNCR
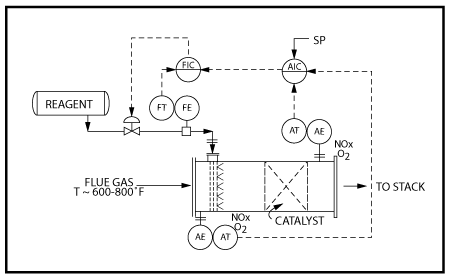
SCR
| Design Criteria | SNCR | SCR |
|---|---|---|
| NOx Reduction Efficiency | 40-75% | 60-90% |
| Temperature Window | 870°-1200°C (1600°-2200°F) |
165°-600°C (325°-1100°F) |
| Reactant | Ammonia or Urea | Ammonia or Urea |
| Reactor | None | Catalytic |
| Waste Disposal | None | Spent catalyst |
| Thermal Efficiency Debit | 0 – 0.3% | 0% |
| Energy Consumption | Low | High I.D. fan |
| Capital Investment Costs | Low | High |
| Plot Requirements | Minor | Major |
| Maintenance | Low | 3 to 5 years (typical catalyst life) |
| Ammonia/NOx (Molar Ratio) | 1.0 – 1.5 | 0.8 to 1.2 |
| Urea/NOx (Molar Ratio) | 0.5 – 0.75 | Not Applicable |
| Ammonia Slip | 5 to 20 ppmvd | 5 to 10 ppmvd |
| Retrofit | Easy | Difficult |
| Mechanical Draft | Not Required | Required |
In this SNCR process, ammonia vapor carried by an air stream or steam is injected into the flue gas at the appropriate temperature zone, 870°-1200°C (1600°-2200°F), effecting a reduction of NOx to nitrogen and water.
The injection of ammonia into flue gas leads to a complexity of intermediate chain branching reactions. The following two simplified chemical equations summarize the overall process:
Equation 1 – 2NO + 4NH3 + 2O2 = 3N2 + 6H2O
Equation 2 – 4NH3 + 5O2 = 4NO + 6H2O
Equation 1 is the NOx reduction reaction which occurs in the 870°-1200°C (1600°-2200°F) temperature range by the injection of ammonia alone. NOx reduction effectiveness can be enhanced down to 700°C (1300°F), by injection of hydrogen (H2 along with NH3). However, as indicated by Equation 2, the injection of NH3 into high-temperature flue gas results in increased NOx formation and is thus counterproductive.
For initial NOx levels of 200 ppmvd or less, NH3/NOx molar ratios of about 1.5 are commonly used.
This SNCR process uses urea, CO (NH2)2 as a reducing agent. It injects an aqueous urea solution into the path of the NOx laden combustion products. The urea thermally decomposes to produce chemical species which react with NOx to form nitrogen, carbon dioxide, and water.
Equation 3 – CO (NH2)2 + 2NO + 1/2 O2 = 2N2 + CO2 + 2H2O
Equation 4 – 4NH3 + 5O2 = 4NO + 6H2O
In Equation 3, it follows that the stoichiometric molar rate of urea relative to NO in the combustion products is 0.5, since one mole of urea potentially has two moles of NH2 available to react with NO. The urea injection process for NOx control is also temperature sensitive. The urea solution, therefore, must be injected in the temperature range of 870°-1200°C (1600°-2200°F).
The NOx reduction efficiency of both SNCR processes depends on the following factors:
- Flue gas temperature in reaction zone
- Uniformity of flue gas temperature in the reaction zone
- Normal flue gas temperature variation with load
- Residence time
- Distribution and mixing of ammonia/urea into the flue gases
- Initial NOx concentration
- Ammonia/urea injection rate
- Heater configuration, which affects location and design of injection nozzles
The selective catalytic reduction process removes nitrogen oxides (NOx) from flue gases by injecting ammonia (NH3) into the flue gas and passing the well-mixed gases through a catalyst bed. NOx reacts with NH3 in the presence of the catalyst to produce nitrogen (N2) and water (H2O) as shown in the following equations.
Equation 5 – 4NO + 4NH3 + O2 = 4N2 + 6H2O
Equation 6 – 6NO + 4NH3 = 5N2 + 6H2O
Equation 7 – 2NO2 + 4NH3 + O2 = 3N2 + 6H2O
Equation 8 – 6NO2 + 8NH3 = 7N2 + 12H2O
Equation 9 – NO + NO2 + 2NH3 = 2N2 + 3H2O
Note that the first reaction for refinery applications generally dominates since 90% of the NOx is NO. A wide variety of available catalysts can operate at flue gas temperature windows ranging from 165°–600°C (325°–1100°F). High NOx reduction efficiencies can be achieved if the parameters such as residence time, space velocity, and the correct temperature window are controlled.
The common ratio for SCR technology is NH3/ NOx molar ratio of 1.0.
What our customers are saying...
"PCC... The best-kept secret in the industry."
— Rohm & Haas
"PCC works like a well-oiled machine."
— Louisiana Pigments Company
"We would not be where we are today without the engineering knowledge and efforts, quality of construction, professionalism, and cooperation of your first-class organization."
— Montauk Energy Capital
"PCC's commitment to safety and quality allow completion ahead of schedule, under budget, and safely."
—
"PCC's desire to deliver a quality product was apparent throughout all phases of our project, and PCC's overall gas combustion experience resulted in a robust and reliable operating unit."
—
"We are very pleased that it was commissioned five weeks earlier than the contract completion date."
—
"PCC was absolutely on time with delivery of our thermal oxidizer... I was quite impressed. All the units we bought from PCC run flawlessly, even after 10 years. We can't calculate a MTBF, because there have been no failures."
—